Organic Rankine Cycle (ORC)
Basics
Organic Rankine Cycle
The Rankine cycle is a thermodynamic cycle widely used by power plants to convert water into steam, then expanding that steam through a turbine to produce mechanical work. The Organic Rankine Cycle (ORC) is a refinement of this technology, using an organic working fluid in place of water. This working fluid, in our case Pentafluoropropane, has a boiling point less than that of water in order to utilize lower temperature heat sources when compared to larger Rankine cycles.
ElectraTherm uses an ORC with proprietary technologies to generate up to 125 kW (with a 250 kW unit in development) of clean electricity from heat sources as low as 70°C. These lower ranges open up opportunities across industries that previously lacked sufficient heat for power generation. Operators take advantage of their excess heat to generate sustainable power – increasing efficiency, reducing energy/fuel costs, and decreasing their carbon footprint.
Our Technology
The centerpiece of ElectraTherm’s ORC systems is a specially-designed twin-screw expander from parent company, BITZER. The implementation of this technology represents a dramatic shift from previous ORC technologies utilizing radial or axial turbines – providing a more cost efficient, robust design that greatly enhances reliability.
Since our systems are using an expander with lower temperature heat, there is less pressure on the pump and lower operating speeds compared to turbine technologies – leading to easier operation and less maintenance. The expander also tolerates “wet” dual-phase flow, allowing it to reliably generate power while accepting variations in both temperature and flow while allowing for a turbine’s worst fear – moisture.
ElectraTherm’s ORC systems are flexible and scalable – which means easy integration and the ability to scale from 75 – 125 kilowatt (or less) to multi-megawatt applications.
Our Process
- Working fluid is pumped to a higher pressure and transferred to the preheater.
- The temperature of the working fluid is increased in the preheater and sent to the evaporator.
- Heat captured by the evaporator boils the working fluid into a high pressurized vapor.
- Vapor flows through the twin-screw expander, spinning an electric generator to produce power.
- The vapor is cooled and condensed back into a liquid and the cycle repeats.
ORC Heat Recovery Benefits
Though applications may not be 100% clean energy, ORC power generation in itself is a 100% clean solution with no emissions or harmful byproducts – hot water is the fuel. Compared to other renewable energy sources, the leveled cost of electricity is extremely low, making it a great value for organizations looking to improve their bottom line while achieving sustainability milestones.
Increase Efficiency
By utilizing excess heat to generate electricity, energy efficiency is significantly improved – up to 10% in some applications – and the cooling load is offset 70-100%. This amounts to a substantial reduction in the consumption of fossil fuels, their related emissions, and operating costs.
Environmental Incentives
Our operators frequently benefit from a range environmental incentives promoting efficiency, clean energy, renewable heat, and more. Depending on where the ORC system is commissioned, you may qualify for attractive kickbacks. For applicable projects this can significantly improve the economics.
Promotes ESG
Innovative technologies – such as heat to power and net-zero cooling to power – that improve operations as well as the environment are the perfect addition to any business. With the world eyeing ways to achieve carbon net-neutrality, organizations that take steps to achieve that goal will distinguish themselves from their competition.
Heat Sources
Applicable fluid heat sources are between 70°C and 150°C.
Gaseous heat sources above 150°C can also be used with the deployment of a secondary heat exchange loop.
Our low-temperature ORC solutions use hot water as fuel in order to generate up to 125 kWe of clean electricity, at no additional cost to the operator. That water can get to us any number of ways, as long as we have enough of it and at a high enough temperature. Power output is directly correlated to the flow rate and source temperature, however an application can get away with a lower flow rate if the water is a high enough temperature.
Common Applications
// Water used for cooling circuits (engines and compressors)
// Micro-geothermal sources (brine, co-produced fluids)
// Industrial waste heat such as hot gases from kilns, furnaces, etc.
// Exhaust and flue gases (requires additional heat exchanger)
// Thermal oil or other high-temperature sources (requires additional heat exchanger)
// Boiler systems such as found in anaerobic digestion and biomass processes
// Net-zero cooling to power (power-generating radiator alternative)
Any application that deals with heat that can be transferred to a fluid can integrate ElectraTherm’s solutions for ORC power generation. For detailed specifications, references, or general information – please reach out to one of our representatives by visiting our Contact Us page.
Performance Variables
Available Heat / Thermal Power
Available thermal power is the raet of BTUs/hr or kWth that is continuously produced by the waste heat source and available to be consumed by ElectraTherm’s ORC power generation systems. The more available heat the more electrical output there is.
Flow Rate
While the heat generated available for conversion plays a large role in system performance, the flow rate of the heat source plays an equally large role. A higher flow rate increasing the thermal energy provided to the ORC. A lower-temperature heat source with a high flow rate can achieve maximum output and vice versa for heat sources with lower flow rates.
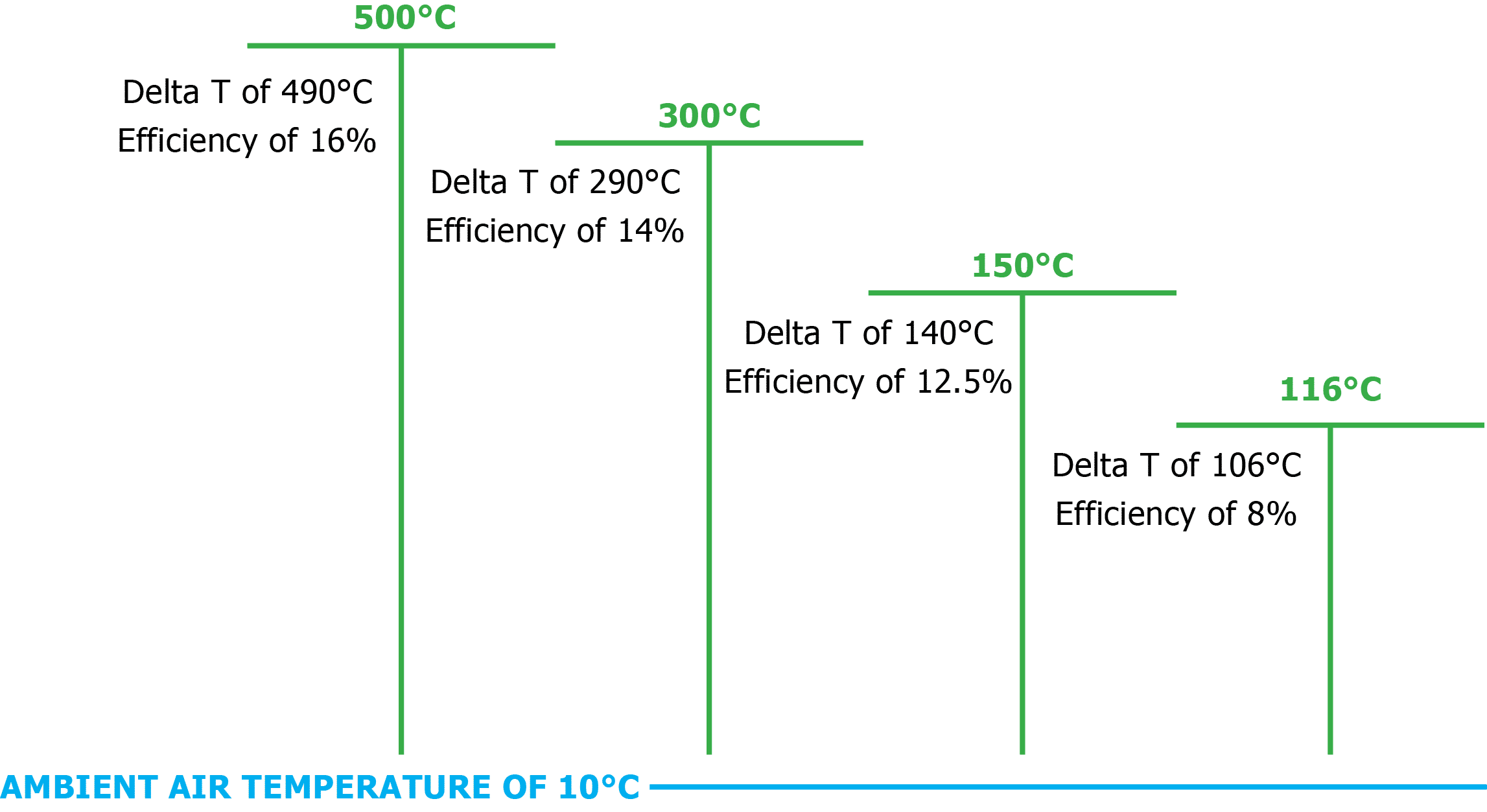
System Delta T
Site conditions such as system delta T (which is affected by ambient air temperature and the temperature differential between the heating and cooling water) also affects ORC performance.
System delta T is the ΔT temperature differential between the hot source and condensing source, and is also the primary driving force behind increased efficiency in ORC systems. The temperature ranges for TH and TC that low grade heat ORC systems typically operate in will dictate lower efficiencies than high grade heat ORC systems because of the higher heat source temperature TH. The Power+ system is limited by delta T temperature because of the physical properties of liquid water.
The ORC system’s location influences the ambient air temperature conditions. In locations with hot climates, such as Africa and the Equator, net power output will be lower than net power outputs produced by machines that are installed in cold climate locations, such as Northern Europe, even at the same hot water input temperature. This discrepancy is due to a lower system delta T (difference between the hot water input and condensing temperature) in hot climates versus that in cold climates.
Heat Engine Example
A heat engine is a simple engine that converts thermal heat into mechanical work. A heat engine operates by extracting heat from a hot reservoir and moving it over to a cold reservoir, generating work in the process.
In order to maximize the amount of work a heat engine can produce, the temperature of the hot reservoir needs to be increased as much as possible, whereas the temperature of the cold reservoir needs to be reduced.
Questions?
Contact us for a conversation about your unique project needs.
Use our project evaluation form to jump start the estimating process.
